|
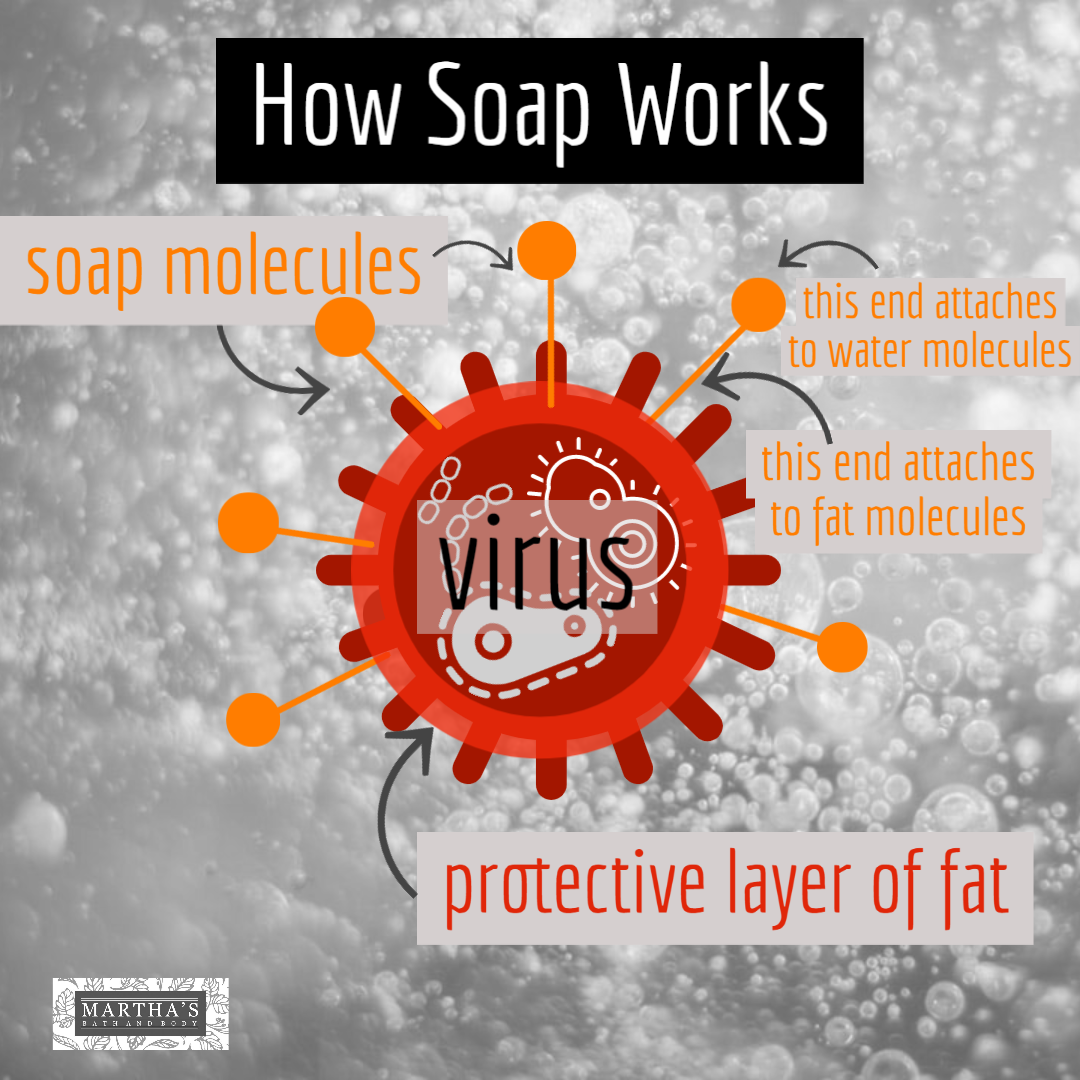
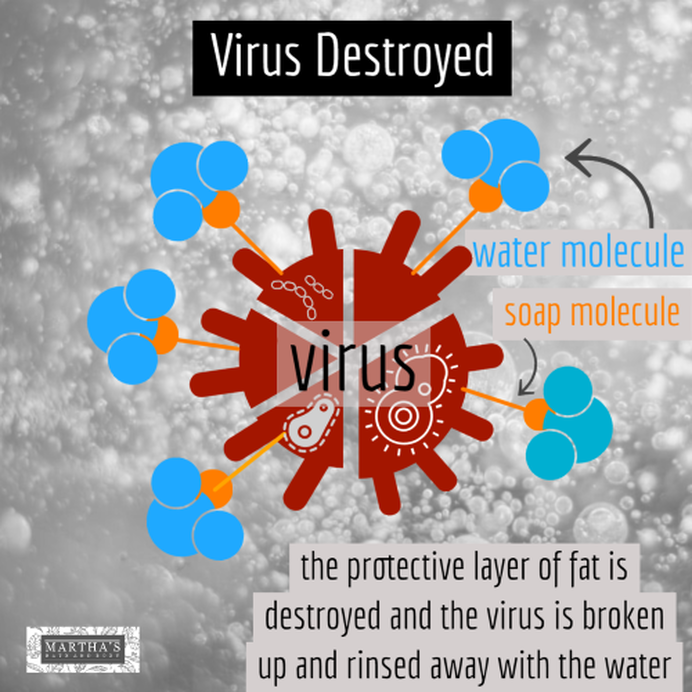
Last month we discussed the differences between soap and detergent. We received some questions from some of you about our soap, so we thought we'd talk a bit about the chemistry of soap and why soap is so effective. We shared a video on our website about this that you can watch here. We should start by saying that when we speak of soap, we are not just talking about our brand, but true soap in general. The soap molecule is unique in its structure. Here's a simplified graphic of a soap molecule. It has two ends -- one end is a negatively charged head which attracts water. The other end is a hydrocarbon chain (or tail) which attracts fats or impurities. The coronavirus has a protective layer of protein and fat. The tail of the soap molecule is attracted to the fat, while the head of the soap molecule is attracted to water. The soap connects the water molecules with the fat molecules and causes the fat molecules to break apart. The soap bonds the impurities with the water, and it is all safely rinsed down the drain when we wash our hands. Detergents work in the same way. Soap kills bacteria or viruses by breaking apart its structure. The soap does not have to have an "anti-bacterial" additive to do this -- in fact, anti-bacterial soaps have not been shown to be any more effective than regular soap, according to the FDA. Be sure to use the soap with warm water, and wash your hands for at least 20 seconds to give it time to work. This applies to any soap, not just Martha's soap. So now you know how soap destroys Covid-19!
0 Comments
Leave a Reply. |
Our PassionThis blog is an interactive place where we can share more about natural skin care and how we create what your skin craves! Archives
November 2022
Categories |



 RSS Feed
RSS Feed